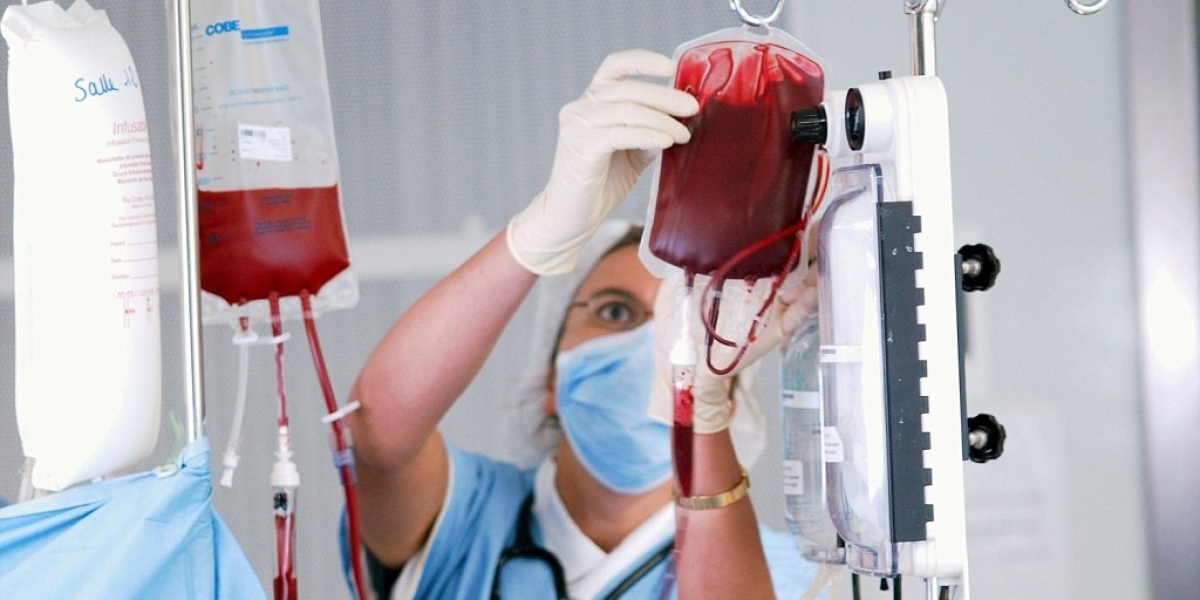
Blood transfusion is a pillar of contemporary medicine, with a key role in surgeries, trauma care, cancer, and chronic disease management. Nevertheless, the lifesaving potential of transfusions has a downside when blood is not screened or matched correctly. It is here that blood transfusion diagnostics becomes crucial. As worldwide healthcare systems remain focused on patient safety and quality of care, the market for blood transfusion diagnostics is increasing in importance fueled by advances in technology, higher demand for blood, and tighter regulative measures.
Blood transfusion diagnostics involve a variety of tests and technologies aimed at confirming the compatibility and safety of donor blood. From typing and screening to infectious disease testing, these diagnostics are critical for precluding transfusion-related complications and for providing patients with safe and effective care.
Get Sample Report- https://www.theinsightpartners.com/sample/TIPRE00005176
Understanding Blood Transfusion Diagnostics
Blood transfusion diagnostics encompasses a suite of laboratory tests and analytical techniques used in blood banks and hospitals. These tests are essential for:
Blood typing: Identifying a person’s blood group (A, B, AB, or O) and Rh factor (positive or negative).
Antibody screening: Detecting unexpected antibodies in a recipient’s blood that might react with donor blood.
Cross-matching: Ensuring compatibility between donor and recipient blood before transfusion.
Infectious disease screening: Viral testing for HIV, hepatitis B and C, syphilis, and other transfusion-transmissible infections.
proper diagnostics minimize the risks of hemolytic reactions, alloimmunization, and disease transmission.
Major Market Drivers
One of the primary drivers of the blood transfusion diagnostics market is the increasing number of surgical procedures and medical emergencies that require blood transfusion. From major surgeries and childbirth complications to trauma and anemia treatment, the need for blood is constant and growing.
Increasing incidence of chronic diseases, including cancer and kidney disease, also accounts for the need for transfusions and, subsequently, diagnostic tests that provide transfusion safety.
Another key driver is the increased focus on ensuring blood safety by healthcare regulatory institutions and organizations, including the WHO and the Red Cross. Regulations and guidelines requiring strict screening for infectious agents and compatibility tests are encouraging hospitals and blood banks to enhance their diagnostic facilities.
Technological innovation is at the forefront of revolutionizing the market. Automated platforms, molecular diagnostics, and next-generation immunohematology platforms are enhancing transfusion diagnostics accuracy, speed, and efficiency, eliminating human error and turnaround time.
Market Segmentation
By Product
· Instruments & Kits and Reagents
By Application
· Disease Screening and Blood Grouping
By End User
· Hospitals
· Diagnostic Laboratories
· Plasma Fractionation Companies
Key Players
· Grifols, S.A.
· F. Hoffmann-La Roche Ltd
· Immucor, Inc.
· Bio-Rad Laboratories Inc.
· Abbott
· Thermo Fisher Scientific Inc.
· Ortho Clinical Diagnostics
· DiaSorin S.p.A.
· Quotient Limited
Geography
· North America
· Europe
· Asia-Pacific
· South and Central America
· Middle East and Africa
Emerging Trends
The market is experiencing a transition towards molecular blood group genotyping, which offers greater insight into antigen profiles and enhances matching, particularly in patients with unusual blood types or chronic transfusion requirements.
Automation and integration are also on the increase. Blood banks are increasingly implementing fully automated platforms that integrate several diagnostic functions—typing, screening, and disease detection—into a single workflow to eliminate workload and maximize throughput.
Point-of-care (POC) testing is also drawing interest, especially in remote or emergency environments. Portable solutions offer immediate results and allow faster decision-making, particularly in field hospitals or disaster areas.
Screening for infectious diseases is becoming more sophisticated with nucleic acid testing (NAT), enabling earlier detection of viral infections compared to the traditional serological tests, thereby further enhancing transfusion safety.
Market Challenges
Albeit substantial progress has been made, market challenges include the high expense involved in advanced diagnostic machinery and test kits, which can be a hindrance to adoption, particularly in resource-limited environments.
Complex regulatory settings and the necessity of adhering to high standards of quality can equally become obstacles for producers and laboratories.
Furthermore, trained workforce shortages and shortages of infrastructure in certain areas affect the provision of quality diagnostic services on a regular basis.
Conclusion
The market for blood transfusion diagnostics is instrumental in providing safe and effective transfusions—a key component of patient care in hospitals across the globe. As healthcare systems develop and expand, the need for accurate, efficient, and quick diagnostic solutions will continue to rise.
Innovations in molecular diagnostics, automation, and infectious disease screening are opening the way for a safer transfusion environment. By solving existing issues and making diagnostics more available in all regions, this market will continue to facilitate the life-saving work of modern medicine.